News
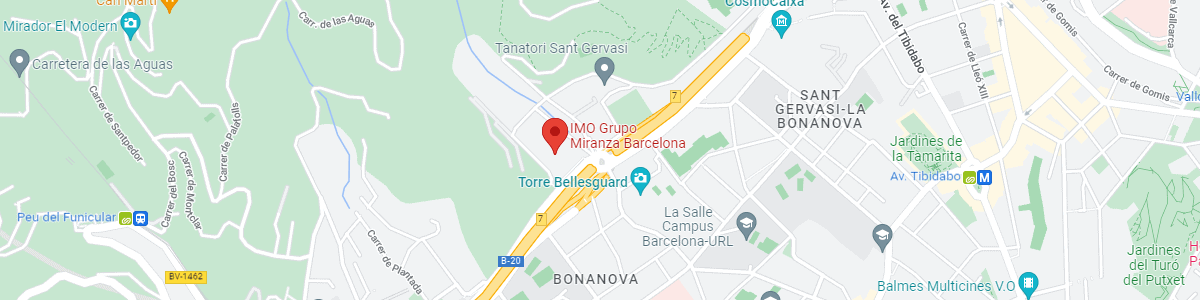
IMO Institute of Ocular Microsurgery
Josep María Lladó, 3
08035 Barcelona
Phone: (+34) 934 000 700
E-mail: international@imo.es
See map on Google Maps
By car
GPS navigator coordinates:
41º 24’ 38” N – 02º 07’ 29” E
Exit 7 of the Ronda de Dalt (mountain side). The clinic has a car park with more than 200 parking spaces.
By bus
Autobus H2: Rotonda de Bellesguard, parada 1540
Autobus 196: Josep Maria Lladó-Bellesguard, parada 3191
Autobuses H2, 123, 196: Ronda de Dalt – Bellesguard, parada 0071
How to arrive at IMO from:
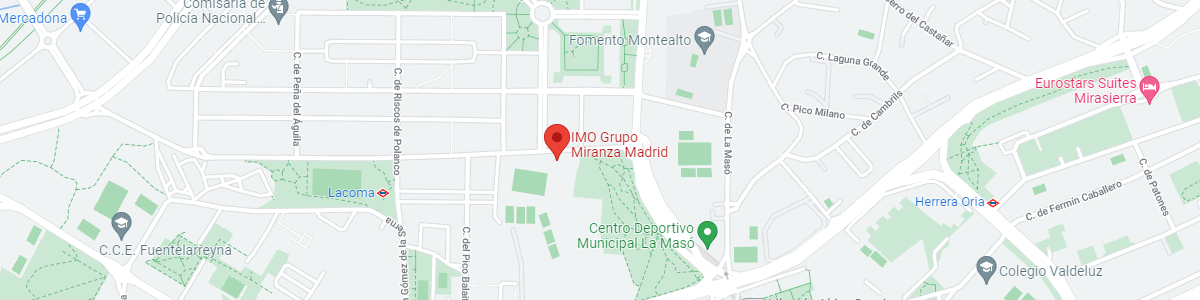
IMO Madrid
C/ Valle de Pinares Llanos, 3
28035 Madrid
Phone: (+34) 910 783 783
See map in Google Maps
Public transport
Metro Lacoma (líne 7)
Autobuses:
- Lines 49 & 64, stop “Senda del Infante”
- Line N21, stop “Metro Lacoma”
Timetables
Patient care:
Monday to Friday, 8 a.m. to 8 p.m.
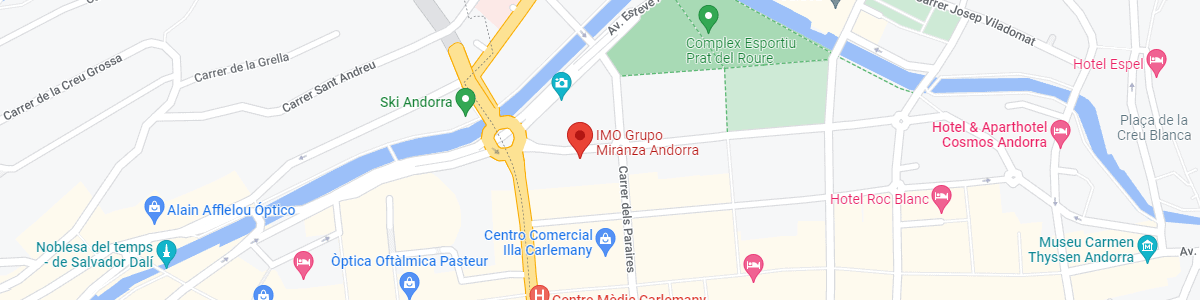
IMO Andorra
Av. de les Nacions Unides, 17
AD700 Escaldes-Engordany, Andorra
Phone: (+376) 688 55 44
See map in Google Maps
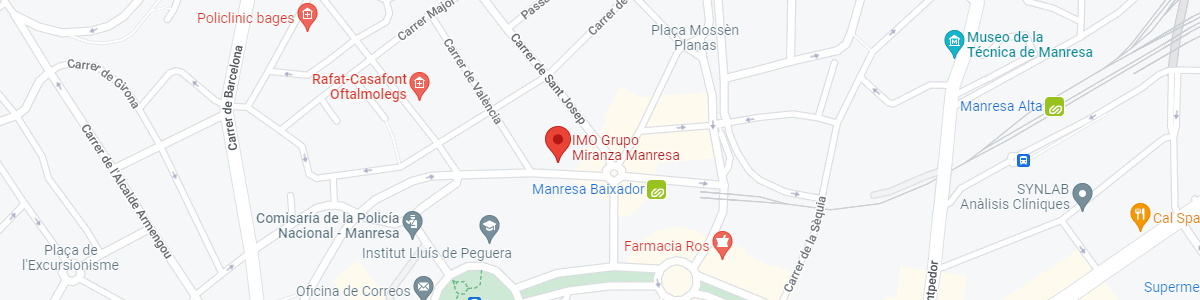
IMO Manresa
C/ Carrasco i Formiguera, 33 (Baixos)
08242 – Manresa
Tel: (+34) 938 749 160
See map in Google Maps
Public transport
FGC. Line R5 & R50 direction Manresa. Station/Stop: Baixador de Manresa
Timetables
Monday to Friday, 08:30 A.M – 13:30 PM / 15:00 PM – 20:00 PM